In developing a thorough site characterization program, it is critical to determine the presence and distribution of DNAPL, vapor, dissolved, and sorbed chemical phases of contamination across the various geologic media. The relative distribution of contaminants between different chemical phases and across geologic media within the primary source zone as well as the more distal plume are important in developing a holistic CSM. Understanding the direction and rate of mass transfer within the system indicates the current trajectory of the source/plume evolution over time, which is a key element of a comprehensive CSM.
A CSM should address the following basic questions:
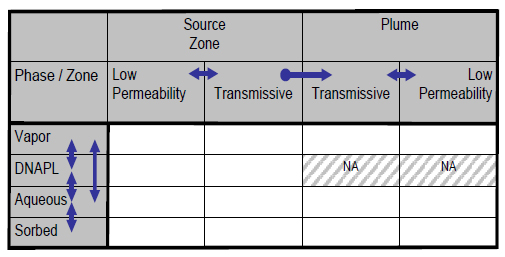
The 14-compartment model (Sale and Newell 2011).
The 14-compartment model (Sale and Newell 2011) is a useful tool for visualizing the distribution and movement of contaminants between the chemical phases that may be present at DNAPL sites (Figure 3-1). The 14-compartment model can help describe the relative locations of contaminants within various subsurface compartments, highlighting the direction in which diffusive mass transfer may be occurring (ITRC 2011b, Section 2.5 and Section 2.6). The 14-compartment model guides the selection of a remedy by showing how contaminants may distribute throughout various compartments, the potential mass transfer (fluxes) among them, and how remediation may or may not equally affect each compartment within the source or plume. The 14-compartment model raises questions about the inclusion of various compartments in the CSM, which in turn influences the data collection objectives and data needs. Thus, it is a powerful tool for site characterization planning and CSM development.
Figure 3-2 illustrates how the 14-compartment model can be used as a characterization planning tool (ITRC 2011b, Table 2-3), showing the early-, middle-, and late-stage plumes described below in
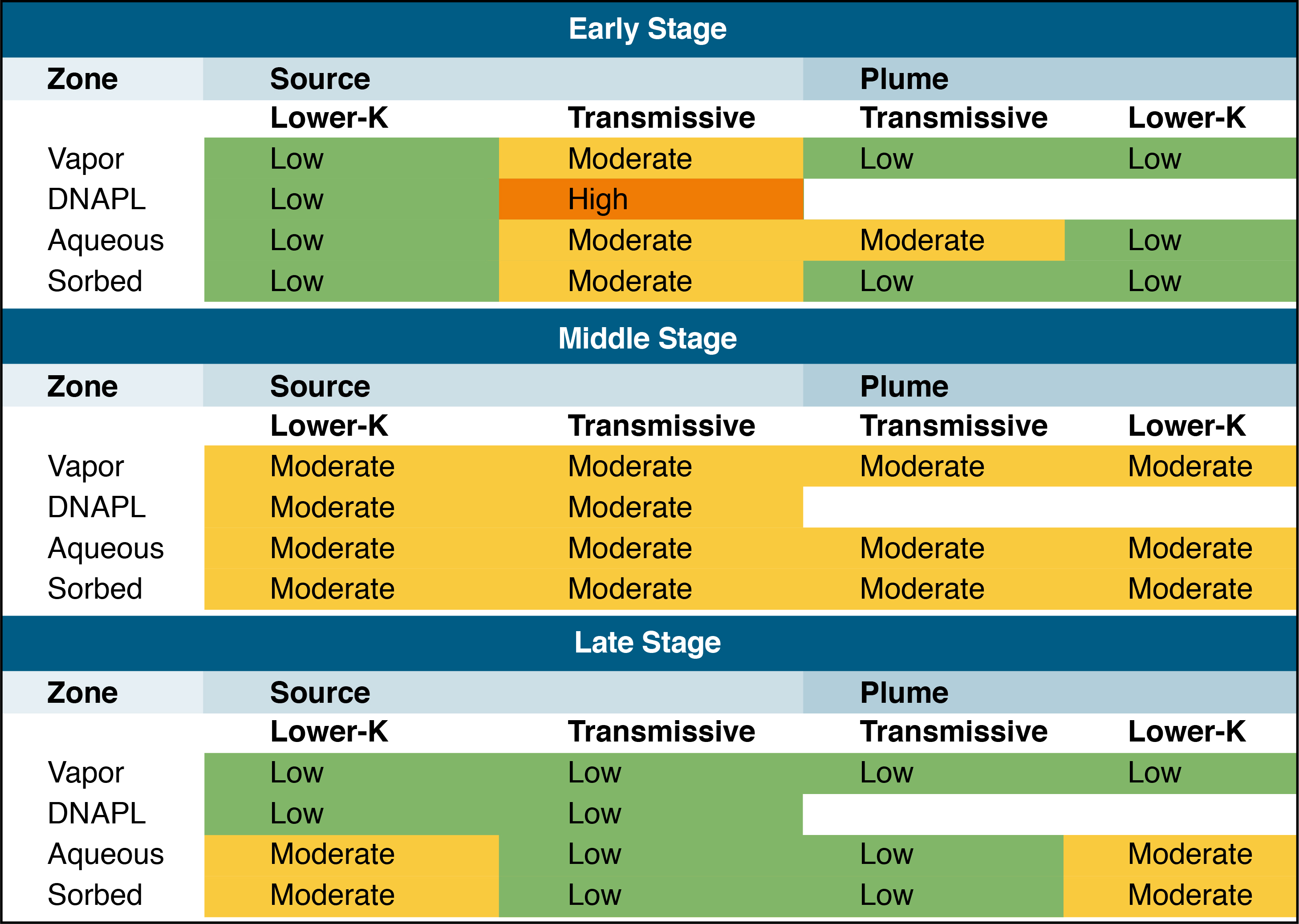
Illustration of the progression of a DNAPL-source zone and associated dissolved phase plume through time that results from mass transfers between compartments, using the 14-compartment model. Groundwater flow (advection) carries contaminant from the source zone into the plume zone, and both diffusive and advective mass transfers can eventually distribute contaminants to all compartments. Over extended periods, contaminants can be diluted to lower concentrations, with stored mass in the lower-permeability zones acting as persistent sources of contamination.
S
Over time, the early-stage DNAPL phase, based on aqueous-phase equivalent concentration, is diminished—by advection, biotic and abiotic degradation, and mass transfer into lower-permeability regions through matrix diffusion and other chemical phases within the source and plume. In the middle stage, the aqueous-phase equivalent concentrations across affected phases and zones are relatively equal. In late-stage plumes, contaminant concentrations have attenuated in the more permeable (transmissive) zones and the larger remaining concentrations reside in the lower-permeability zones within both the source and the plume (
These concepts are a useful part of site-specific CSM development because plume maturity has a major effect on the response to treatment of a source and plume, and therefore on the potential efficacy of possible remediation efforts. Understanding the age of a site and the compartments that require investigation (and to what degree and resolution) is critical in identifying data needs, developing effective data collection objectives, and selecting appropriate site investigation/data management tools. Appendix E presents a screening method for estimating whether a chlorinated solvent release is in its early, middle, or late stage.
DNAPL behavior in the subsurface includes processes related to its transport; interphase chemical mass transfer into aqueous, sorbed, and vapor phases; and degradation reactions. Barring any remedial measures, natural degradation reactions generally occur slowly. Chlorinated solvent, coal tar, and other DNAPL aqueous solubilities are relatively low, and DNAPL mass transfer to the aqueous phase is typically limited by diffusive processes, either at the pore scale or at the scale of stratigraphic (for example, sand and silty sand interbeds) or bedrock (sandstone to siltstone interbeds, layered volcanics, fractured metamorphic rock) heterogeneities. As a result, DNAPLs may persist in the subsurface for long periods (several decades or more), depending on their site-specific solubility, type, mass, and distribution, as well as geologic conditions.
The presence of DNAPL constituents represents a potentially persistent reservoir of contaminant mass that can continue to release dissolved contaminants over long periods; thus, understanding the potential presence and distribution of DNAPL in the subsurface is critical to long-term environmental site management. Characterizing sites contaminated with DNAPLs must take into account the subsurface behaviors of DNAPL and subsequent phases, including the physics of DNAPL migration that control three-dimensional distribution and dissolved-phase contaminants.
With its primary goal of extracting oil (that is, NAPL) from the subsurface, the petroleum industry has conducted exhaustive research on the dynamics of immiscible fluids and the importance of subsurface permeability architecture (stratigraphic or in fractured media). In the 1980s and 1990s, the wealth of understanding originating from the field of petroleum reservoir geology began to be applied to environmental releases of DNAPLs. As it relates to DNAPL transport and behavior in porous media and multiphase flow, this information is summarized below.
DNAPL migration is governed by scientific principles of multiphase flow in porous media. The study of the simultaneous flow of multiple immiscible fluids originated from the fields of soil irrigation science (Richards 1928) and petroleum engineering (Muskat 1937), and there is nearly a century of scientific literature and understanding in this area. The application of multiphase flow concepts to water resources and the DNAPL problem was pioneered by Schwille (1988), and early reviews of the relevant immiscible fluid concepts were provided by Corey (1986), Mercer and Cohen (1990), Cohen and Mercer (1993), and Pankow and Cherry (1996).
Downward migration of DNAPLs is largely driven by gravity (that is, downward density flow); however, if a continuous body of DNAPL develops, pressure head can be transmitted from overlying DNAPL. For n
Capillary entry pressure is the key property governing DNAPL penetration into a given subsurface media. It is significantly influenced by the subsurface matrix (including pore throat size and heterogeneity) and the interfacial tension between the groundwater and the specific DNAPL constituents. Because DNAPLs are generally nonwetting and hydrophobic, greater pressures are required to displace groundwater and enter lower-permeability (smaller-pore-sized) media. Therefore, DNAPLs migrate generally downward until they encounter materials with pore sizes too small to penetrate, at which point they spread laterally and potentially accumulate until enough pressure is created to allow the DNAPLs to displace the groundwater and penetrate the pore throat. Cohen and Mercer (1993), Pankow and Cherry (1996), and Payne et al. (2008) provide more discussion on DNAPL migration into or through low-permeability layers.
Subsurface lithologic heterogeneity leads to differences in subsurface pore structure and capillary properties. As a result, downward migration of DNAPL results in flow instability where isolated fingers of preferential DNAPL migrating along preferred pathways develop, leaving a highly variable distribution. These variations are commonly present in the subsurface matrix, even in formations that initially appear to be homogeneous. The existence of highly variable fingers of DNAPL in the subsurface is a key factor, making detection of DNAPL challenging, and leading to the need for improved site characterization methods.
After cessation of the release, as a DNAPL migrates through the subsurface, it leaves a residual trail, which is essentially immobile unless subsurface pore pressures change due to disturbance of the matrix or other activities. There may also be higher-than-residual-saturation zones of DNAPL that are not actively migrating—for example, where thin lenses of DNAPL are perched above low-permeability zones. While these zones are potentially mobile if disturbed, they are isolated; they do not have the ability to enter the smaller pore spaces as separate phase liquids and will remain in place unless subsurface conditions change. Although residual DNAPL is considered immobile under normal subsurface conditions, it can act as a long-term source of dissolved-phase groundwater contamination. As groundwater moves through the subsurface and contacts isolated DNAPL ganglia and pools, the DNAPL slowly dissolves into the groundwater based on its effective solubility. Residual DNAPL in the vadose zone also acts as an ongoing source for soil gas contamination, vapor migration, and potential vapor intrusion.
The perspective of what represents a source zone at a DNAPL site has evolved in recent years. In the 1990s and early 2000s, source zones were considered to be the areas affected by DNAPL phase contamination; however, the current recognition that contaminant mass (sorbed or dissolved phase) can be stored in lower-permeability zones within the plume body has broadened that definition.
This evolution in understanding the extent of source material reflects the increasing recognition that, at many sites, DNAPL is not the primary factor in contaminant mass that sustains a plume over time. Especially at late-stage sites, the contaminant mass stored in low-permeability zones within the plume body acts as a primary source to help sustain the dissolved plume through back-diffusion processes. Thus, many of the improved characterization methods described in this document focus on delineating the geologic heterogeneity and contaminant mass distribution across different geologic units and across different contaminant chemical phases.
As discussed in
Most chlorinated solvent DNAPL sites can be described in terms of three stages of development (Sale et al. 2008; Sale and Newell 2010; Stroo et al. 2012, ITRC 2011b), as discussed below.
In the initial (early) stage, DNAPL predominates and contaminants migrate downward through pore spaces of more transmissive zones of the geologic formation, leaving ganglia of residual (immobile) DNAPL held within the pores by capillary forces. If an initial release is large enough, DNAPL may pool on top of low-permeability zones. Contaminants also begin to partition into soil gas and groundwater, forming dissolved-phase and potentially vapor plumes. Contaminants also sorb to soil and sediment surfaces. This process occurs more readily in the more transmissive zones of a formation, but mass also begins to transfer to less permeable zones.
In the middle (mature source) stage of the source zone DNAPL life cycle, a significant portion of the contaminant mass has migrated into vapor, aqueous, and sorbed phases in both transmissive and lowest permeability zones of the formation. The DNAPL phase may not enter the lowest permeability zones of the formation; instead, dissolved-phase contaminant molecules diffuse into the groundwater in the lowe
In the late (weathered, aged/treated sources) stage of chlorinated solvent sites, DNAPL is often no longer detected and chlorinated solvent concentrations may persist in the aqueous phase of the transmissive zone due to desorption and back-diffusion from the contaminant mass in the low-permeability matrix into the higher-permeability matrix. The maturation process is influenced by a number of factors, including the amount of initial release, solubility of the contaminant in water, groundwater flow velocity, and architecture of the transmissive and low-permeability zones of the formation (Sale et al. 2008).
These three stages are illustrated in Figure 3-3. Appendix F presents a newly developed screening method for estimating whether a chlorinated solvent site is in its early, middle, or late stage.
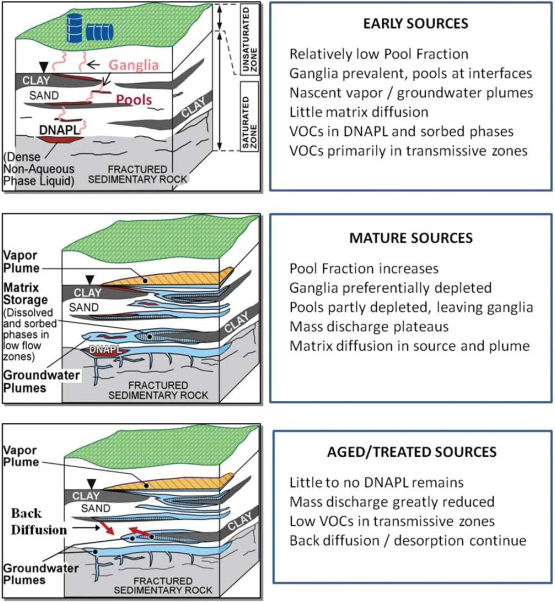
Evolution of a DNAPL source/plume (Stroo et al. 2012).
Figures 11 and 12 in Sale and Newel's work (2011, pp 28-29) illustr
DNAPL life cycles at coal tar and creosote vs. chlorinated solvent sites can be similar, but can also be significantly different due to varying chemical and physical properties. Because coal tar and creosote DNAPLs may have significantly lower solubility and higher viscosities than chlorinated solvents, the early phase of the DNAPL life cycle often dominates at coal tar and creosote sites.
Historically, it was common for very large amounts of coal tar and creosote DNAPLs to be disposed of or released over time in industrial areas. These contaminants often saturated the subsurface and either fully or partially displaced groundwater; thus, it is not uncommon to find several feet of coal tar or creosote DNAPL in monitoring wells and DNAPL-saturated portions of the subsurface. Depending on the NAPL saturation, these DNAPLs may be mobile or immobile. At sites where the release is old and no longer occurring, although DNAPL saturations may be high enough to be mobile, the gradients no longer exist that could drive migration unless site conditions are disturbed. The DNAPL-saturated portions of the subsurface also prevent or significantly limit groundwater flow through a site because of decreases in relative permeability due to the presence of dissimilar fluids. The limited groundwater flow and low solubility of these fluids may limit the amount of dissolved-phase contamination and lengthen the occurrence of DNAPL.
Coal tar DNAPL released at any site can also have significant physical and chemical differences over time.
Including the DNAPL life cycle and matrix diffusion in a CSM has several important implications for initial site characterization and remediation-based characterization activities. Several key differences between a DNAPL-centric and DNAPL-plus-matrix-diffusion site characterization programs are discussed in following sections.
While low-solubility DNAPLs such as coal tar and creosote may remain as separate phase compounds in the subsurface for decades, dissolution may largely remove the separate phase compounds of chlorinated solvent DNAPLs from the subsurface; however, when matrix diffusion is considered, indirect measurements of DNAPL may actually be false positives. In such cases, DNAPL may no longer be a key part of the CSM, and continued attempts to locate DNAPL by indirect measurements may be addressing the wrong source material (Parker, Gillham, and Cherry 1994; Parker, Chapman, and Guilbeault 2008).
A modeling study (Seyedabbasi et al. 2012) showed site characterization results (50-year-old, 675-kilogram release of TCE) consistent with late-stage sources. In late-stage sites, the contaminant mass in low-permeability matrices serves as the primary source material, resulting in elevated dissolved-phase concentrations for a longer period than the DNAPL itself. The simulated TCE source in the model, DNAPL ganglia in a series of small pools, persisted for about 40 years, whereas back-diffusion from low-permeability zones maintained the average plume concentration above drinking water standards for at least an additional 83 years.
This modeling effort supports the DNAPL source zone life cycle model, illustrating a hypothetical site at which actual DNAPL persisted for only a few decades before being dissolved away. Dissolved-phase mass stored in lower-permeability zones supplanted DNAPL as the source through the action of matrix diffusion, and was able to maintain a dissolved contaminant plume above drinking water standards for an even longer period after all DNAPL was removed from the system.
A modified form of Fick’s Law is typically used to describe diffusion in unconsolidated deposits and porous bedrock (Parker et al. 2003).
Where JD is the diffusive flux (grams/[cm2-sec]), h is the porosity (dimensionless), D* is the effective diffusion coefficient (in centimeters per second [cm/s]) and dC/dx is the concentration gradient (in g/cc). D* is usually less than the free solution diffusion coefficient (Do), which ranges from 10-5 to 10-6 cm/s for most chemicals, because of the tortuosity of the porous media. This modified form of Fick’s Law indicates that the porosity of the low-permeable media is an important factor in evaluating diffusive flux. The concentration gradient, the driving force for diffusion can vary by orders of magnitude; however, the surface area over which the diffusive flux occurs can be an even more important factor in contaminant mass transfer and plume behavior. For example, in fractured porous media such as fractured sedimentary rock and fractured clayey deposits, the contaminant mass in fractures is exposed to a large surface area of rock such that diffusion-driven mass transfer of the contaminant mass into the matrix may be large.
At sites where high-solubility DNAPLs have been released, the contaminant mass may have been transferred to lower-permeability media, which is now generating a dissolved-phase contaminant plume without actual DNAPL remaining in the source zone. For such sites, determining the contaminant distribution, identifying the geologic media with the greatest contaminant mass, and measuring the physical properties of this media (contaminant concentration, media porosity, and permeability) can be more useful than trying to confirm or refute the presence or absence of DNAPL in the subsurface; however, DNAPL that remains at many sites across North America is a continuing source of contamination, creating and sustaining dissolved plumes and vapor plumes. In summary, if DNAPL has not been identified at a site, it may not exist; however, when it is found at a site, it must be included in the CSM.
While it may be difficult to prove that DNAPL is present, there are proven techniques for evaluating the presence of matrix diffusion sources. A scoping approach and a detailed site characterization approach are described below.
A scoping approach relies on source history, hydrogeology, and contaminant type to indicate if significant matrix diffusion sources could be present. Sale et al. (2008) identify the following general conditions driving matrix storage effects:
While these factors can be used in a qualitative sense, other tools can be used to determine semi-quantitative effects of matrix diffusion. For example, the Matrix Diffusion Toolkit is a public domain software tool that can help site personnel estimate what effects matrix diffusion will have at their site. The software contains two different matrix diffusion models, both relying on a simple two-layer conceptualization (a low-permeability layer and a transmissive layer), and a two-phase timing structure: (1) a loading period (when contaminants diffuse from the transmissive zone into the low-permeability zones); and (2) a back-diffusion period (when the low-permeability zone acts as a source to the transmissive zone). The software was developed to assist site personnel in updating or creating a more accurate CSM, which will enable them to determine whether matrix diffusion processes are significant enough to cause rebounding groundwater concentrations in a downgradient plume at concentrations above remediation goals after plume remediation or isolation is complete.
A field-oriented approach, on the other hand, relies on detailed site characterization techniques such as those described by Chapman and Parker (2005). These techniques have the following key elements:
continuous coring (for example, at intervals of a few centimeters) the low-permeability zones to determine the permeability architecture (for example, bedding thickness and sediment composition), and the presence and vertical distribution of contaminants that have diffused into low-permeability zones
The field-oriented approach can provide strong evidence of the mass of contaminants in low-permeability zones, and more importantly can link the location of the contaminant plume to diffusion from the low-permeability zones. For example, matrix diffusion sources are indicated if the low-permeability zone has a shark-fin type vertical contaminant distribution. Present-day soil concentration vs. penetration depth profiles can provide insight into expected diffusion patterns as well as the style of the past concentration history. For example, a shark-fin pattern indicates diffusion gradients from the interior of low-permeability zone to the interface (see Location B in Figure 3-4).
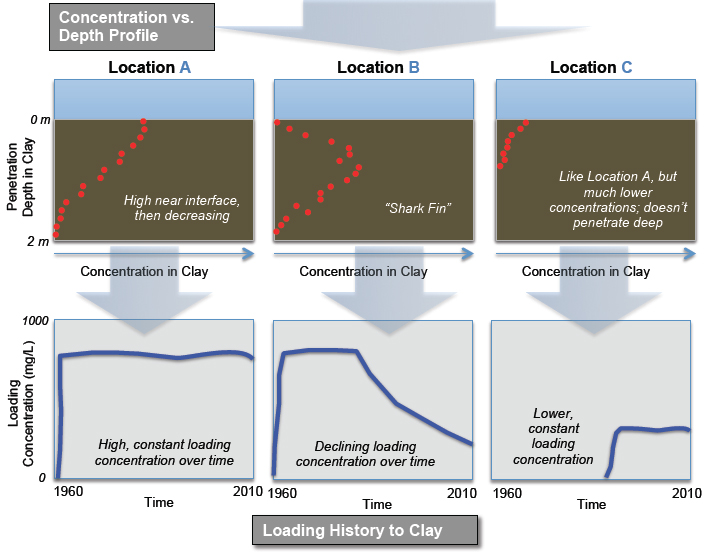
Examples of
As described in
Initially, DNAPL penetrates into bedrock and migrates generally downward through fracture networks (Figure 3-5). The DNAPL continues to migrate until the driving head in the DNAPL has been dissipated and it essentially becomes immobile. The DNAPL gradually dissolves into the groundwater within fractures as well as within the rock matrix porosity in contact with the DNAPL. The dissolved-phase constituents in the groundwater diffuse into the rock matrix porosity. The relative permeability within fractures increases as DNAPL saturation decreases, and water saturation increases as more and more of the DNAPL is dissolved. The dissolved-phase material migrates with the water flowing through the fractures, forming a plume downgradient of the release area. As this plume migrates, molecular diffusion occurs from the plume within the fractures to the matrix porosity.
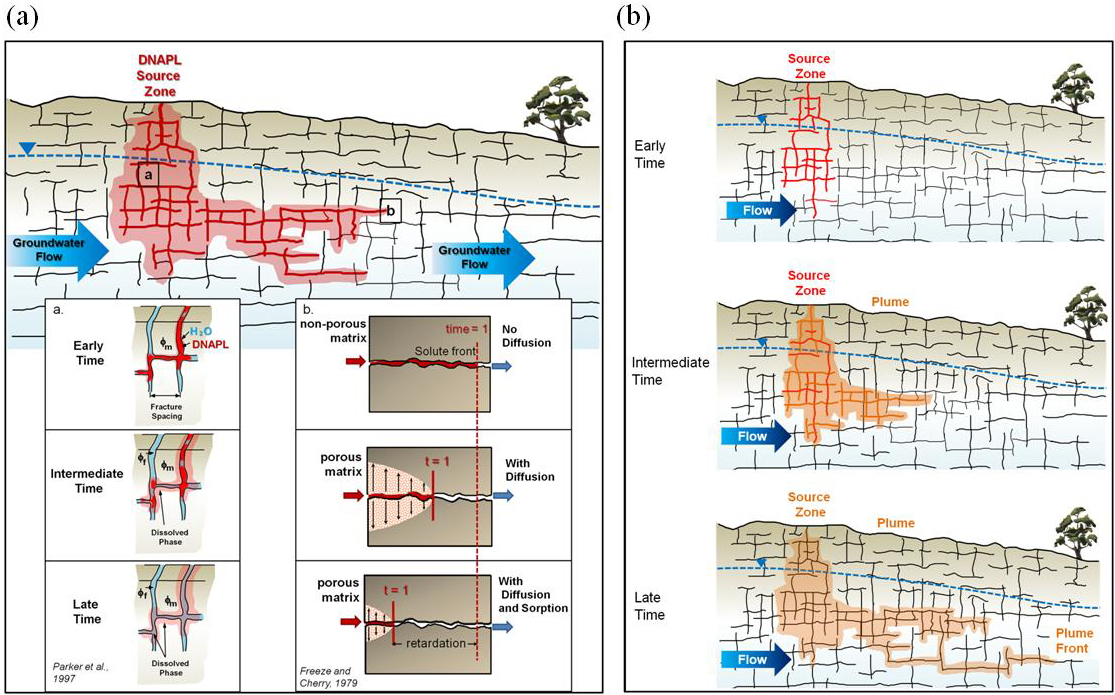
Illustration of fate and transport of DNAPL in a fractured rock environment through time showing distinctly different types of contaminant transport, including DNAPL movement and dissolution, dissolved-phase advection through fractures, and diffusion into the rock matrix. (a) Initially, DNAPL migrates vertically through the fracture network until the initial driving head is dissipated and the DNAPL becomes immobile. The DNAPL begins to dissolve into the water in the fractures and diffuse into the rock matrix, and over time migrate with water flowing through the fractures. These processes together with sorption will retard contaminant migration. (b) This is a conceptualization of the effect of these processes on source zone and plume evolution.
Source: Parker et al. 2012.
If significant matrix porosity is present within the bedrock, DNAPL dissolution is relatively enhanced and, in later stage releases, the DNAPL may dissipate near the release area such that there is no distinction between the dissolved-phase plume areas and the former release area. The dissolved-phase contaminants that have diffused into the matrix porosity may then back-diffuse from the rock matrix porosity into groundwater within the fractures. This may sustain elevated concentrations in the zone formerly containing DNAPL as well as the dissolved-phase plume within the fractures where dissolved contaminants have diffused into the matrix.
Sedimentary rock typically has significantly larger primary porosity than igneous or metamorphic rock, with a notable exception of a variety of volcanic rock. Therefore, DNAPL usually dissipates sooner in sedimentary rock because of its larger matrix storage capacity. The matrix storage capacity can be enhanced significantly due to contaminant sorption, particularly in higher fraction of organic carbon (foc) rock types (mainly siltstones and shales, but all sedimentary rocks can have high foc).
Figure 3-6 illustrates characteristic fracturing for sedimentary and igneous rock types. In sedimentary rock, the fractures are predominantly horizontal along bedding planes, which typically occur at a larger frequency than vertical fractures that intersect at generally right angles. Igneous rock fractures tend to be more chaotic in orientation because of the lack of bedding planes. Igneous rock typically has lower rock matrix porosity than sedimentary rock.
The fracturing and porosity characteristics of crystalline rock present a lower potential for matrix diffusion; however, fault zones or shear zones within igneous or metamorphic rocks can provide laterally extensive two-dimensional features of high permeability and porosity that can behave as rapid migration pathways.
As stated above, Figure 3-5 illustrates the fate and transport of DNAPL over time in sedimentary bedrock. DNAPL initially observed in fractures may dissipate through the combined mechanisms of matrix diffusion and dissolution into groundwater within fractures. In the resulting later stage, the source area may then exhibit back-diffusion from the rock matrix porosity into groundwater within the fractures, but DNAPL would not be observed. In contrast with sedimentary bedrock, DNAPL that migrates into fractures of igneous rock with its characteristic chaotic fracturing and limited rock matrix porosity is more likely to be present as actual DNAPL at older sites (Parker, Gillham, and Cherry 1994).
Due to the nature of consolidated media environments (for example, bedrock), drilling of individual borings may require higher-capacity equipment and thus involve higher cost; however, characterization is still practical. Additionally, in contrast to the approach applied to unconsolidated media, fewer core holes may be drilled in consolidated media, with greater reliance on higher-resolution borehole tools to characterize the geology, hydrogeology, and contaminant distribution within and between holes. Given the complexities associated with tool selection, Chapter 4 is dedicated to this topic and discusses tools applicable to both unconsolidated and consolidated media.
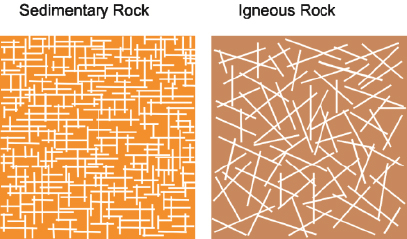
Schematic of fracture orientation in sedimentary and igneous rock
Source: Figure courtesy of Patryk Quinn.
It is necessary to define the types, size, orientation, frequency, and distribution of fractures when characterizing contaminant distribution and flow in fractured bedrock, as they create the primary contaminant advective flow paths. A variety of tools are available to help characterize bedrock fractures. Not all fractures are hydraulically connected; during characterization, it is therefore important to evaluate fracture connectivity using tools that allow methods such as cross-hole testing to gain an understanding of the potential flow paths for groundwater movement and to obtain information for gradient calculations. If a fracture or set of fractures are not directly connected to the contaminant transporting fractures, these fractures are less significant from a contaminant migration perspective.
Some fractures that may connect to contaminant-bearing fractures may dead end and not contribute significantly to the advective transport of the dissolved-phase contamination. Such dead end fractures can, however, provide significant surface area and be important sinks for matrix diffusion.
The primary challenge when characterizing fracture networks is determining their role in contaminant transport (that is, advection or diffusion). For this evaluation, it may be useful to test discrete portions of a borehole or single fracture opening, and to monitor in depth-discrete portions of nearby holes and within other portions of the hole being tested. Information gained by connectivity tests on entire boreholes may not provide the discrete fracture transport pathway information required to adequately characterize the contaminant plume. Multiple tests and development of a collaborative data set will also be required to provide the data necessary to understand contaminant flow and distribution. Fracture transmissivity, orientation, and hydraulic interconnectivity data are critical for developing rigorous CSMs in fractured rock environments.
Naturally occurring organic carbon (measured as foc) may be found lining fractures (secondary porosity) or may occur within the rock matrix itself (that is, within the matrix or primary porosity). The occurrence of organic carbon within the fractures, or matrix of a particular fractured bedrock, can play a significant role with regard to fate and transport and ultimately the remediation of DNAPL, for the following reasons:
Consistent with site characterization objectives, foc should be analyzed for fractured bedrock, although not all fractured bedrock is equally likely to exhibit organic carbon. The occurrence of organic carbon is possible at sites underlain by sedimentary, igneous, or metamorphic rock; however, it is more likely to occur in sedimentary rock, which is more likely to exhibit matrix porosity. Where the site characterization objectives include gaining an understanding of the fate and transport and providing a basis for ultimately remediating the site, and where DNAPL and dissolved contamination occurs within fractured sedimentary rock, analysis of foc should always be performed. Similarly, analysis of foc should be performed for sites where DNAPL or dissolved contamination occurs within fractured igneous or metamorphic rock that additionally exhibits primary porosity due either to the rock type (for example, slate, phyllite, schist, and gneiss, for which foliation is common) or the use of other site characterization tools that indicate the occurrence of primary porosity. For metamorphic rock in particular, foliation, microfractures, and the degree of crystallization can result in primary porosity, and such rocks should be evaluated for foc.
As discussed in this document, it is clear that low-permeability materials in the subsurface, such as fine silts and clays, may impede vertical DNAPL migration. In some cases, however, the presence of a clay layer may not fully protect groundwater below. Many clay deposits in the subsurface are not pure clay and do not resemble fully plastic modeling clay. Variations in grain size and composition and depositional environments create the same heterogeneities that affect DNAPL migration and groundwater flow in coarser materials.
There may also be significant secondary porosity. Clays are commonly fractured, especially in the unsaturated zone. Fractures in the clay create preferential pathways for advection of DNAPL and dissolved-phase contamination, and create new surfaces for matrix diffusion to occur. Plants may create significant preferential pathways as they grow and their roots advance through the subsurface. Depending on the depositional history, a clay deposit may be small with minimal lateral extension. Past erosional events may have also removed portions of the clay, creating direct pathways through it. The act of investigating a site may also create pathways through clays when boreholes are not fully sealed or when sealed with a material that is incompatible with the site-specific DNAPL (McCaulou and Huling 1999).
When DNAPL and bentonite or other material are used to grout a borehole, some DNAPLs such as TCE or creosote can desiccate bentonite clay, facilitating the creation of cracks that significantly increase hydraulic conductivity (McCaulou and Huling 1999). If this occurs where DNAPL is resting on a low-conductivity clay layer, any cracks that form due to desiccation of the clay by the DNAPL may act as preferential pathways for DNAPL migration through the clay.
Additionally, according to the SERDP-ESTCP ER-1737 Fact Sheet (Abdul et al. 1990):
Low permeability zones in the subsurface contain clay and previous research with landfill liners suggests that contact between nonchlorinated organic liquids can cause the clay structure to compress, creating macropores in the clay and resulting in an increase in hydraulic conductivity of up to five orders of magnitude. If such a process occurs with chlorinated DNAPLs in the subsurface, then diffusion into the low-permeability regions may be enhanced, and advection, which is usually considered negligible, may also be important. Furthermore, waste DNAPLs contain surfactants. If these surfactants diffuse into the low-permeability materials and sorb to the mineral surfaces, the materials may become organic-wet, resulting in active imbibition of the DNAPL.
Considering clay intervals as impermeable to DNAPL and associated aqueous compounds can often result in erroneous predictions and underestimation of fate and transport. Careful characterization of the clay content and mineralogy of these lower fine-grained layers helps to account for physical disturbance and chemical reactions that may provide avenues for DNAPL transport.